Immunology expert Prof Adrian Liston elected Fellow of the Academy of Medical Sciences
 Wednesday, May 12, 2021 at 10:58AM
Wednesday, May 12, 2021 at 10:58AM - Professor Adrian Liston is one of 50 new researchers elected as Fellows of the Academy of Medical Sciences.
- Candidates’ scientific achievements are peer reviewed, with successful researchers selected based on their contribution to advances in human health and welfare.
- In a career spanning continents and disciplines, Prof Liston’s key scientific findings have expanded our understanding the human immune system as it interacts with our own bodies during health and disease.
Professor Adrian Liston, Senior Group Leader in the Immunology programme, has been elected a Fellow of the Academy of Medical Sciences for his pioneering research in immunology and neuroimmunology. Fellows of the Academy of Medical Sciences are elected for exceptional contributions to the medical sciences either in the form of original discovery or of sustained contributions to scholarship.
Professor Dame Anne Johnson, President of the Academy of Medical Sciences, said: “I am truly delighted to welcome these 50 new Fellows to the Academy’s Fellowship, and I offer my congratulations to each of them on their exceptional contribution to biomedical and health science. The knowledge, skill and influence that each brings to the Fellowship is the Academy’s most powerful asset.”
Commenting on his election, Prof. Liston said: “This is a really wonderful recognition of the quality of the science being run by my team here at the Institute. I am honoured to work with the best team of immunologists around, always willing to explore new fields and push the boundaries forwards.”
Prof. Liston’s work at the Institute explores uncharted areas of immunology with large implications for human health. The current research interests of the lab include working to shed light on the interactions between the immune system and the brain, and to learn more about how immune cells adapt and operate in different tissues around the body.
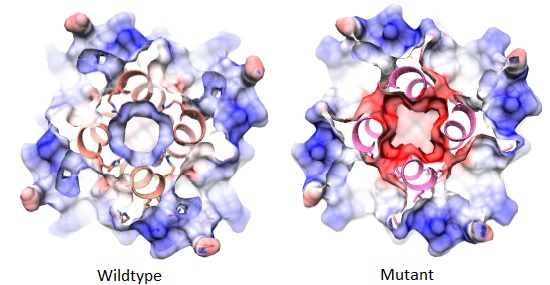
Exciting recent findings include that brain-resident T helper cells act to support the development of microglia and that the presence of these cells in the brain is essential for normal brain development in mice. These findings open up avenues of investigation that may help to drive the development of new therapeutics for neurological injuries like stroke and traumatic brain injury, and raise intriguing questions about the role of immune cells in information transfer between the body and the brain.
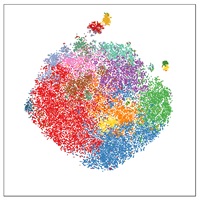
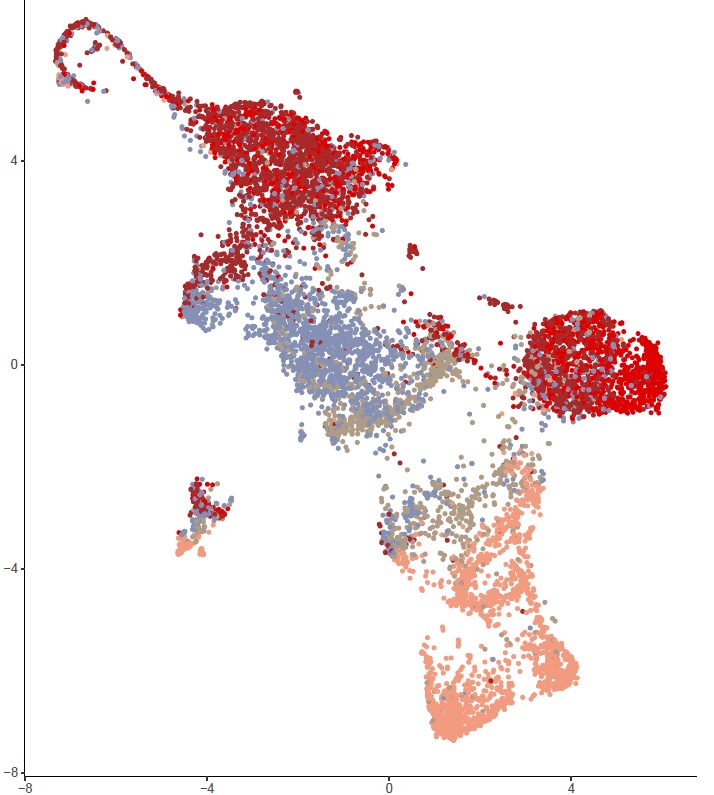
Prof. Liston’s expertise in immune system profiling has been applied to understand the factors that shape our immune system; looking at the factors that drive immune system variation between individuals, applying machine-learning and immune-profiling to improve the diagnosis of juvenile idiopathic arthritis in children, and a small-scale study to dissect the immune characteristics of severe COVID-19 responses.
“I am delighted to congratulate Adrian on his election as Fellow of the Academy of Medical Sciences,” said Dr Martin Turner, Head of the Immunology research programme, “Adrian’s work has been pivotal in increasing our understanding of autoimmunity and T cell populations, his recognition by the Academy is well deserved. Since joining the Institute, Adrian has proved himself to be an excellent leader, facilitating the international exchange of ideas, and promoting shared practices and values between his labs.”
Prof. Liston joined the Babraham Institute in 2019, after 10 years of running a research laboratory in Belgium. His team has expertise in cellular immunology, neuroimmunology, diabetes, immunodeficiency and systems immunology, and the team takes a creative and multidisciplinary approach to extending our understanding of the immune system.
After gaining his PhD with Professor Chris Goodnow at the Australian National University studying T cell tolerance and diabetes, Prof Liston moved on to study regulatory T cells with Professor Sasha Rudensky at the University of Washington before starting his own lab at VIB in 2009. Prof. Liston has produced over 180 publications with over 10,000 citations and has been awarded two ERC grants, the Eppendorf Prize and a Francqui Chair, among other honours.
Beyond academic publications, Prof. Liston also works to engage a wider audience with his research, in particular the importance of vaccination to protect health. In 2020, he published two children’s books, ‘Battle Robots of the Blood’, and ‘All about Coronavirus’ to explain the coronavirus pandemic in an accessible way to children. He has also drawn on his own experience to offer advice to early career researchers looking to advance in academia.
A celebratory event in July will bring the Academy’s new Fellows together for a virtual induction and a series of talks from new members.